Treatment Process
About CAR-T Cell Therapy
CAR-T cell therapy is a targeted, personalized therapy that contains patients’ autologous T cells reengineered to fight cancer. KYMRIAH® (tisagenlecleucel) is a CAR-T cell therapy genetically modified to identify and eliminate CD19-expressing malignant and normal cells. Upon binding to CD19-expressing cells, the CAR transmits a signal to promote T cell expansion, activation, target cell elimination, and persistence of the KYMRIAH cells.1
Prior to KYMRIAH CAR-T cell therapy, pediatric and young adult patients with r/r ALL had limited treatment options and low survival rates.2 Planning ahead and identifying the right patient for KYMRIAH is critical in optimizing CAR-T treatment outcomes.
| 5-year survival rates are ≤40% after allogeneic stem cell transplant (SCT) in children who were in their second or later remission2 |
ALL, acute lymphoblastic leukemia; CAR-T, chimeric antigen receptor T cell; r/r, relapsed/refractory.
Patient Identification
Consider KYMRIAH for pediatric and young adult patients with any of the following clinical characteristics1:
Have not gone into remission following frontline treatment (primary refractory)
Have relapsed and cannot achieve remission (chemorefractory)
Have had second or subsequent relapse following complete remission or SCT
MORE THAN 5000 CLINICAL AND COMMERCIAL PATIENTS HAVE RECEIVED KYMRIAH3
As soon as you have identified a patient who may be a candidate for KYMRIAH, contact a treatment center to discuss eligibility, current treatments, and next steps, as ongoing chemotherapy can lead to T cell depletion,4 which may affect the quality of the final cell product. The treatment center physicians will coordinate with you to ensure the appropriate patients receive KYMRIAH therapy. For more information on the initial KYMRIAH process, download the Starting Your Patients on KYMRIAH guide.
KYMRIAH therapy is available at select treatment centers across the United States. Please call KYMRIAH CARES™ at 1‑844‑4KYMRIAH (1-844-459-6742) for more information about KYMRIAH Treatment Centers, the ordering process, product information, and patient support. Find a KYMRIAH Treatment Center
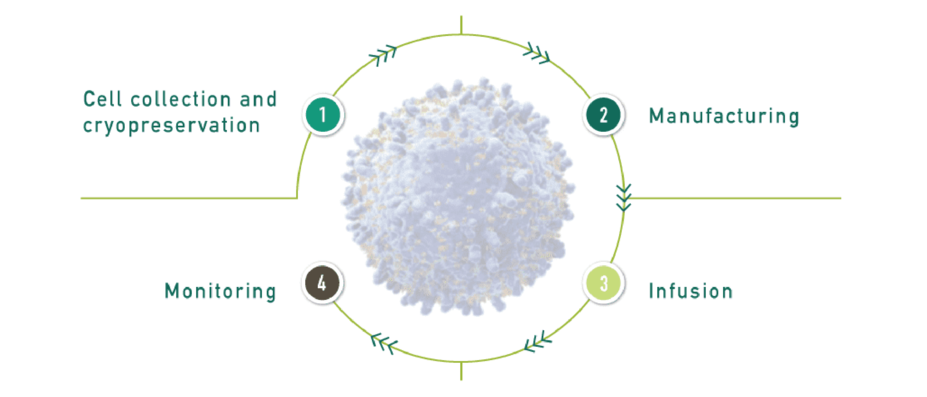
Steps for Patients to Receive KYMRIAH
Cell Collection and Cryopreservation Leukapheresis, when a patient’s own T cells are collected from the blood, occurs over 3 to 6 hours. Within 24 hours, the leukapheresis material is cryopreserved.1,5,a | |
Manufacturing The patient’s cryopreserved cells are shipped via specialized courier to the Novartis FDA-approved manufacturing facility, where the patient's cells are genetically reprogrammed into KYMRIAH CAR-T cells. | |
Infusion The patient may receive lymphodepleting chemotherapy to prepare the body for KYMRIAH CAR-T cells.1 The patient receives their reprogrammed KYMRIAH CAR-T cells during a single infusion. KYMRIAH can be administered in either an inpatient or outpatient setting at the treating physician’s discretion. | |
Monitoring Monitor the patient 2 to 3 times during the first week following infusion. The patient should stay within proximity of their KYMRIAH Treatment Center for at least 4 weeks after KYMRIAH infusion to monitor and be treated for potential side effects.1 Routine long-term monitoring is recommended. Patients should be informed about, and encouraged to participate in, the KYMRIAH registry. |
aLeukapheresis material from a previous collection may be used if it was collected within 30 months at a Novartis-approved apheresis collection facility.5
The KYMRIAH CAR-T Cell Therapy Manufacturing Process
THE KYMRIAH MANUFACTURING PROCESS UTILIZES CRYOPRESERVATION, WHICH ALLOWS FOR:
Flexibility for patient scheduling5 | |
Optimal timing for leukapheresis5 | |
Shipping assurance6 | |
Storage of excess cells5 |
bKYMRIAH is individually manufactured for each patient. It is important to keep in mind that not all patients’ cells can be successfully manufactured into KYMRIAH CAR-T cells.